Chemical Compounds that interfere with our environment
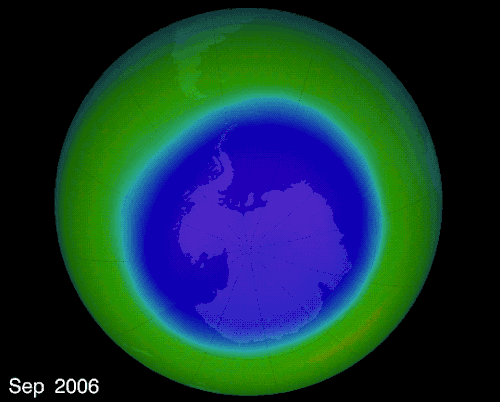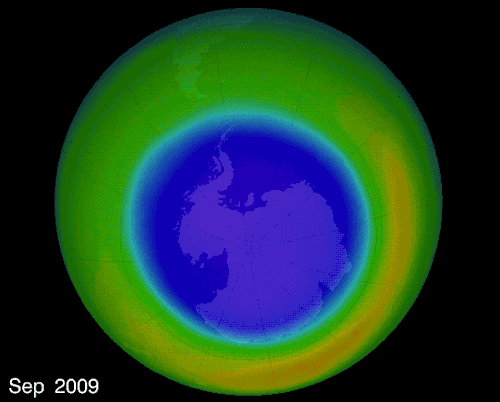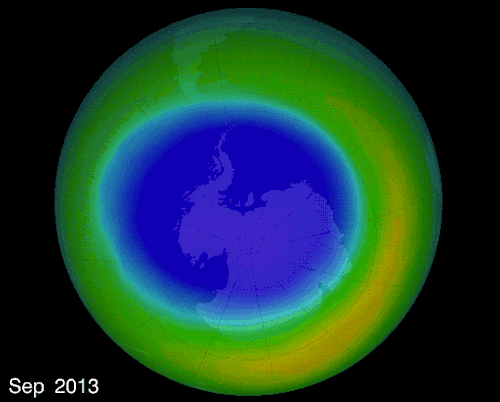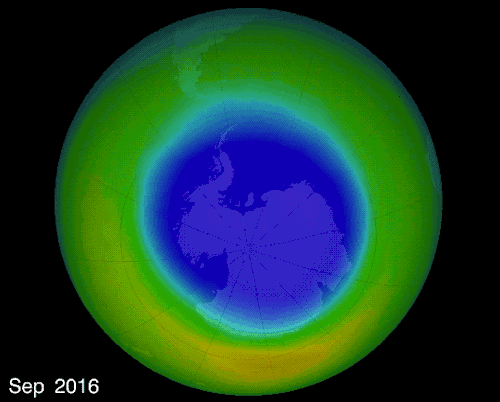
THE OZONE HOLE IN EARTH’S ATMOSPHERE OVER ANTARCTICA
Elements and chemical compounds have been circulating within our environment for billions of years (since Earth was created 4.5 billion years ago). Throughout this vast amount of time chemicals in our environment have circulated regularly (Ex. Nitrogen), however circulation of certain chemicals can be disrupted which leads to drastic changes on planet Earth.
Too much or too little of a chemical compounds in our environment can disrupt both abiotic and biotic components of the environment. This includes (but is not limited to):
disrupting life cycle of living things
affecting the climate
affecting u.v radiation levels on Earth
changing animal & plant populations
disrupt pH of environments
Pollution:
Any substance introduced into an environment that has harmful or poisonous effects.
Toxicity of chemical compounds
TOXINS:
Substances that produce serious health problems or death when introduced into an organism.
Lead (toxic to animals and plants)
Arsenic (very toxic to animals)
TOXICITY:
Describes how poisonous a substance is.
LD50:
A measurement used to compare toxins. LD means lethal dose, ‘50’ represents 50%.
LD50 is the amount of a substance that causes 50% of a group of test animals to die if they are given a specified dose of the substance all at once.
The more toxic a substance is, the lower the LD50 number is.
DIFFERENT TYPES OF CHEMICALS AFFECT ORGANISMS IN DIFFERENT WAYS:
Theobromine is found in chocolate:
LD50 humans : 1000 mg/kg
LD50 cats : 200 mg/kg
LD50 dogs : 300 mg/kg
This compares the toxicity of different substances because it is comparing the dosage that will produce death.
Question: If a bar of dark chocolate has 810 mg of Theobromine and your dog who weighs 5 kg eats it, what will happen?
MEASURING small CHEMICAL concentration
PARTS PER MILLION...(YAY MATH)
SHARKS HAVE A KEEN SENSE OF SMELL - SOME SHARKS CAN SMELL ONE DROP OF BLOOD IN 1000 ML OF WATER
Some toxins are so toxic that only a small amount can affect organisms. We measure these tiny concentrations in PPM.
PARTS PER MILLION:
Measures concentration dissolved in a million units of a substance (round to the nearest whole number)
PPM = (Amount of Solute / Amount of Solution ) x 1,000,000
Solution = Solute + Solvent
1g water = 1ml water
FOR EXAMPLE….
If you have a container with one million marbles, and one of those marbles is red, then we could say there is one part per million of red marbles.
use parts per million (ppm) or milligrams per litre (mg/L)
one part per million means that one unit of an element or chemical can be found in one million units of solution.
EXAMPLE 1
If you had 25 pieces defective in a shipment of 1,000 pieces.
Then 25/1000= .025 or 2.5% of that shipment is defective.
Now calculate that percentage by one million. 0.025 X 1,000,000 = 25,000 PPM.
EXAMPLE 2
Suppose you make a food colouring solution by putting 99 mL of water in a beaker and adding 1 mL of food colouring.
The concentration of food colouring in this beaker is 1 part food colouring per 100 parts solution, which is 1% or 0.01.
Now calculate that percentage by one million. 0.01 X 1,000,000 = 10,000 PPM.
EXAMPLE 3:
Suppose I have 100g of a salt solution, and the solution has 205 PPM of NaCl (Salt). How many grams of salt is there in the solution?
First write out 205 ppm in a g/1,000,000 format = 205g/1,000,000g.
Now multiply the ratio from step two by the amount of solution in step one. 100g x 0.000205 = 0.0205g
In the 100g of salt solution there is 0.0205g of NaCl present.
Note: parts per billion (ppb) and parts per trillion (ppt) exist but the use of special equipment is necessary and only extremely hazardous substances are measured to this level, for example PCB’s.
examples of ppm
CO2 IN ATMOSPHERE
Dissolved Oxygen IN WATER
The type and amounts of invertebrates found in water are determined by the amount of dissolved oxygen.
5 mg/L (= 5ppm) of dissolved oxygen supports most organisms
OXYGEN LEVELS IN WATER AT DIFFERENT TEMPERATURES
LEVELS OF DISSOLVED OXYGEN DEPENDS ON MANY THINGS:
Temperature - colder water holds more oxygen
Turbulence of Water - water flowing mixes water and oxygen, wind mixes water with oxygen as well
Photosynthesis - underwater plants and algae create oxygen
Number of organisms - more organisms using dissolved oxygen reduces oxygen levels
Nitrogen and Phosphorous - high concentrations of phosphorus and nitrogen increase algal blooms which can lead to a reduction of oxygen levels
ALGAE BLOOMS AFFECTS DISSOLVED OXYGEN
Algae grows on top of lake in warm temperature
Water and atmospheric oxygen are not in contact and can not mix.
Algae dies and the organic matter is now food for bacteria, decomposition uses up more oxygen. Oxygen decreases, fish and aquatic insects cannot survive.
Water Pollution
Water pollution occurs in all bodies of water, from ponds, to lakes, to seas and oceans.
Common Water pollutants include:
1.HEAVY METALS (HG, AS, PB)
Metals that have a density of 5g/cm3 or more, meaning they are 5 times or more heavier than an equal volume of water.
Includes mercury, copper, zinc, lead, cadmium and nickel.
These metals occur naturally and are made into everyday items like tires, batteries, and some fertilizers.
Affect the normal development in children, as well as causing permanent brain damage or death.
2.PLANT NUTRIENTS (N, P, K)
Runoff from fertilizers increase the growth of algae and green plants.
3.PESTICIDES
Some pesticides have long-term effects, but most are designed to last only one growing season, to be broken down by bacteria therefore are no longer toxic.
Many cause pesticide-resistant pests to develop and quickly, because of their rapid reproduction
4. SALTS (NACL, MGS)
Salts used to de-ice sidewalks and roads
Chlorine in salts can harm freshwater organisms
5. ACIDs
From earlier, rain and snow have a normal pH of 5.6 because of CO2 in the air dissolving in them
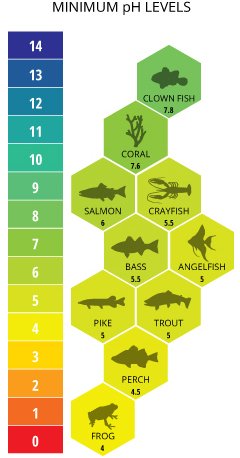
As acidity increases, organisms decrease, for example, most fish disappear when the water’s pH fall to 4.5
SPRING ACID SHOCK :
When acidic deposits build up in ice and snow during winter.
Once it melts in spring, the acid meltwater flows into aquatic systems lowering its pH level.
This affects microbiological organisms, and increases how much heavy metal can dissolve in water.
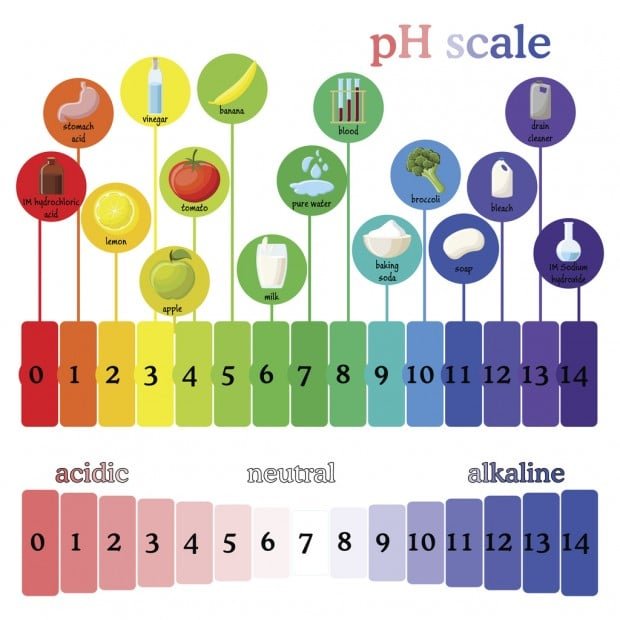
What is pH?
DRAW OUT THE pH SCALE, DON’T FORGET TO LABEL STRONG AND WEAK ACIDS, AS WELL AS STRONG AND WEAK BASES
pH measures the amount of hydrogen ions (H+) in a solution, a solution with many hydrogen (H+) ions is said to be acidic, a solution with few hydrogen ions (H+) is said to be basic. (This is a ‘mickey-mouse’ definition for grade 9, in reality it is a bit more complex than this, but it will do for grade 9!)
ACID:
A compound that dissolves in water to form a solution with a pH lower than 7.
Example : lemon juice
BASE:
A compound that dissolves in water to form a solution with a pH higher than 7.
Example : bleach
PH SCALE:
The pH number of a solution indicates its acidity; it is a measure of the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in a solution.
pH range is from 0 to 14
pH of 1 is very acidic; for example battery acid
pH of 14 is very basic or alkaline; for example household ammonia
pH of 7 is neutral (neither an acid or a base); for example water
The difference between one number and the next on the pH scale represents a 10-fold difference (logarithmic scale) meaning that a solution with a pH of 9 is 10 times more basic than a solution with a pH of 8. Also, a solution with a pH of 2 is 10 times more acidic than one with a pH of 3
What is the pH of Milk, Orange Juice, Bleach, Vinegar, Salt Water, & Cola?
How much more acidic is solution 1 (pH 2) than solution 2 (pH 5)?
MEASURING PH
1. PH METER :
A probe attached to a meter. You submerge the probe in the liquid and get a pH reading on the meter.
2. ACID-BASE INDICATORS:
substances that change colour when they are placed in solutions.
blue litmus paper turn red in an acid
red litmus paper turns blue in a base
3. UNIVERSAL INDICATOR :
A mixture of indicators that change colour over a wide pH range. You add a few drops of the indicator and compare its colour change with the colour chart to determine its pH.
Ocean acidification
Most marine life builds its skeletons with two chemical building blocks: calcium (Ca²⁺) and carbonate (CO₃²⁻)
Carbon dioxide in the air is absorbed by water to form carbonic acid (H₂CO₃), this lowers the pH of ocean water (ocean pH has dropped from 8.2 to 8.1 since the industrial revolution).
Although carbonic acid is considered to be a weak acid, it’s H+ bond better with carbonate than Ca²⁺. This reduces available carbonate for sea creatures and dissolves their shells/skeletons.
NEUTRALIZATION
MMMM … CALCIUM CARBONATE
NEUTRALIZATION:
Is the reaction between an acid and a base that produces water and salt (neutralization products)
Acid + Base → Ionic Compound (salts) + Water
ex. HCl + NaOH → NaCl + HOH
Strong Acid + Strong Base = pH 7 (water + salt)
Strong Acid + Weak Base = Weak Acid
Strong Base + Weak Acid = Weak Base
Weak Acid + Weak Base = pH 7 (water + salt)
LAKE LIMING:
Lakes can be neutralized by adding lime (calcium hydroxide). The acidic water and the basic lime react forming water and salt (neutralization products)
The formation of ACID RAIN
Acid rain is formed by the following:
When sulfur dioxide combines with water to form sulphuric acid (H₂SO₄)
When nitrogen oxides combine with water to form nitric acid (HNO₃)
When carbon dioxide dissolves in water to form carbonic acid. (H₂CO₃)
This water than falls as acid rain.
Ordinary rainwater is slightly acidic with a pH of about 5.6, rain that has a pH less than 5.0 is considered acid rain.
Signs in nature HELP assess water quality
DIVERSITY:
The number of different species in an ecosystem.
CHEMICAL INDICATORS:
Indicators that assess the quality of water, such things as dissolved nutrients (N,P,K), dissolved gases (O2) and dissolved metals (Hg, Cu, Fe, etc.)
OCEAN ACIDIFICATION SLOWLY DISSOLVES PLANKTON SHELLS
BIOLOGICAL INDICATORS:
Organisms that indicate the quality of water, this includes fish, plants, worms, insects, plankton (microscopic algae and tiny animals), protozoa, bacteria and viruses.
MICROBIOLOGICAL INDICATORS:
Microscopic organisms like bacteria can cause serious health problems when present in large enough numbers.
AQUATIC INVERTEBRATES:
Are animals without backbones that live in water
insects
crustaceans (shrimp)
worms
molluscs (clams)
plankton
These organisms are commonly used as biological indicators because they indicate different conditions of the water, for example, oxygen levels, temperature and pH.
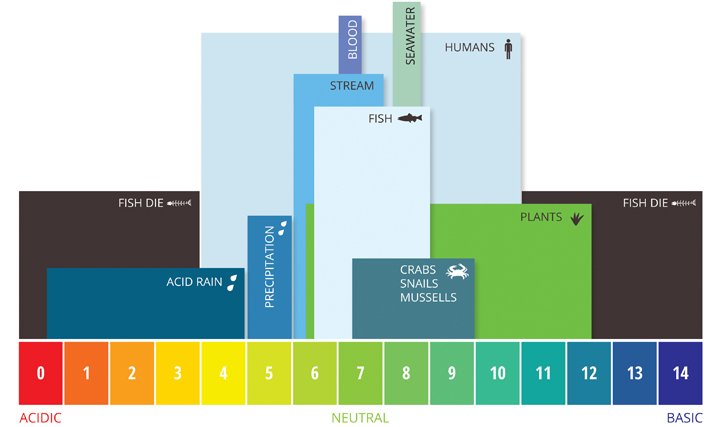
AQUATIC ENVIRONMENTS
Diversity of organisms decreases as pH increases and dissolved oxygen decreases.
Water Guidelines
RHONE RIVER MEETS ARVE RIVER, ARVE IS FED BY GLACIERS, WHILE RHONE IS FED BY A LAKE
Cloudiness in lakes is caused by increase in algal growth, which reduces the oxygen content of the lake affecting organisms that live there.
Clarity is not a good indicator of water quality because some chemicals and organisms cannot be seen with the naked eye. Also some water is more turbid than others.
Water quality is determined according to the use of the water. Water quality guidelines can be based on the following five uses of water:
Human drinking water - highest water quality, no harmful chemicals, no bacteria
Recreation such as swimming - some harmful chemicals, no bacteria
Livestock drinking water - no harmful chemicals, some bacteria
Irrigation - possibly some chemicals, some bacteria
Protection of aquatic life - many dissolved chemicals and bacteria
Effluent:
Liquid waste or sewage discharged into a river or the sea - It can be treated or untreated
AIR pollution - Toxic chemical gases
MANY HARMFUL CHEMICALS ARE RELEASED FROM BURNING COAL FOR ELECTRICITY AND SMELTING OF METAL
Air quality is determined by:
measuring the levels of pollutants in the air
estimating the amount of emissions from pollution sources (forest fires, industrial plants)
SHEERNESS COAL BURNING POWER PLANT — LOCATED SOUTH OF HANNA AB. — IT BURNS COAL TO CREATE ELECTRICITY FOR NEARBY COUNTIES AND CITIES.
SULFUR DIOXIDE (SO2)
Forms when oxygen combines with sulfur
Causes smog and acid rain
Affects the respiratory system (throat and lungs) and irritates the eyes
Major source is burning coal for electricity, industrial processes such as refining oil, and smelting
SCRUBBERS:
Are used to reduce SO₂ emissions by 99%. SO₂ reacts with limestone that converts it to gypsum used in manufacturing.
COMBUSTION VEHICLES ARE A MAJOR CONTRIBUTOR TO NOX BEING RELEASED INTO EARTH’S ATMOSPHERE
NITROGEN OXIDES (NOX)
Formed when nitrogen monoxide gas (NO) reacts with oxygen to form NO2, a toxic brown gas
Causes smog and acid rain
Affects the respiratory system (throat and lungs) and irritates eyes
Sources include combustion reactions that burn coal, oil, natural gas - industrial processes, smelting metal, kilns for cement and brick.
EVEN HOME FURNACES CAN RELEASE CARBON MONOXIDE - MANY HOUSEHOLDS HAVE A CARBON MONOXIDE ALARM INCASE CARBON MONOXIDE LEVELS GET TOO HIGH
CARBON MONOXIDE (CO)
Forms when there is not enough oxygen to produce CO₂, it has no colour and no odour, thus called the ‘silent killer’
Affects the amount of oxygen content carried by blood cells, causing headaches, sleepiness, chest pains, brain damage and death
Sources include combustion in vehicles, wood, natural gas, industrial processes and cigarette smoking
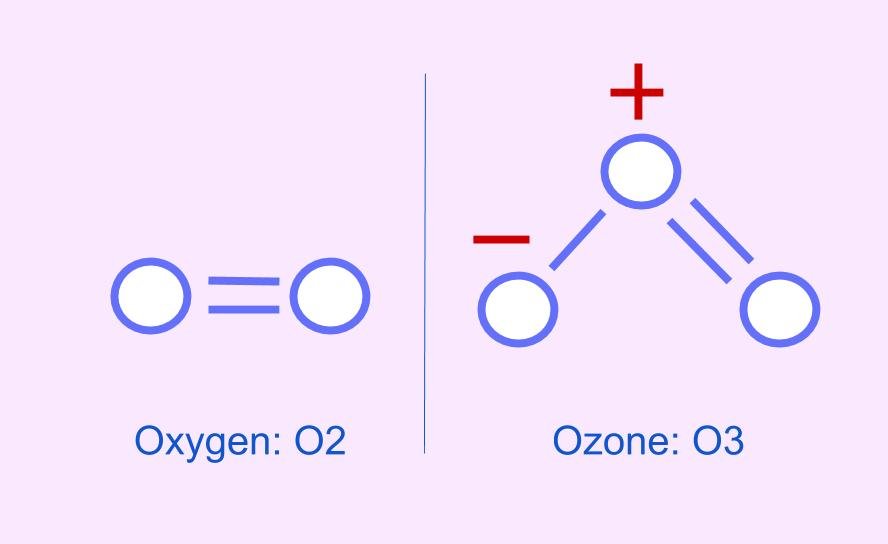
GROUND-LEVEL OZONE (O3)
GREAT IN THE SKY, BUT NOT NEARBY - OZONE IS O₃, WHILE IT HELPS BLOCK UV RADIATION IN THE SKY, ITS REACTIVITY MAKES IT TOXIC TO LIVING THINGS
When nitrogen oxides react with volatile organic compound (VOCs - organic chemicals that evaporate easily) react with oxygen in the presence of heat and sunlight, to create a a colourless, odourless gas called Ozone.
Affects people who have lung diseases (ex. asthma), breathing problems and long-term lung damage especially for developing children
Affects crops (wheat, soybeans, onion) and can causes rapid deterioration in plastic
Sources include fuel combustion in vehicles and industry
Air Pollution - Greenhouse Gases
ATMOSPHERIC POLLUTION EFFECTS THE ENTIRE GLOBE!
CLIMATE CHANGE(d):
A change in global or regional climate patterns
GREENHOUSE GAS:
Atmospheric gases that traps infrared (Heat) from being emitted back into space (much like a blanket for Earth)
CO₂ (Carbon Dioxide)
CH₄ (Methane)
NOx (All Nitrogen Oxides, the ‘x’ indicates a number, it could be NO₂, NO, NO₃, N₂O, etc.)
H₂O (Water vapour)
SUNLIGHT RADIATION HAS ENOUGH ENERGY TO ENTERS THROUGH EARTH’S ATMOSPHERE, HOWEVER AFTER BOUNCING OFF THE SURFACE OF THE EARTH THE RADIATION IS TRAPPED INSIDE THE ATMOSPHERE - THIS RAISES GLOBAL TEMPERATURES.
THE GREENHOUSE EFFECT :
Atmospheric gases in the air trap heat from the Sun’s radiant energy (we want this to an extent)
A GREENHOUSE EFFECT OUT OF CONTROL
With more gases trapping more heat, the Earth’s temperature is increasing
GLOBAL WARMING:
An increase in the average global temperature
Water vapour and CO₂ are the 2 main contributors to global warming
Leads to climate change, violent storms, flooding, heat domes, polar ice caps melting, sea level rise, greater amount of storms with higher intensity, crop loss, habitat loss, and the spread of disease
Caused by human activities and natural events (volcanic eruptions, forest fires), increasing the concentration of greenhouse gases
A direct result of global warming is the melting of many polar ice caps around the world. (Bloomstrandbreen glacier (Norway) before and after, 1936 vs 2009)
Air Pollution - Ozone depletion
OZONE LAYER:
A layer of ozone (O3) gas that resides in the upper atmosphere surrounding Earth
Absorbs ultraviolet radiation (UV) protecting the Earth’s surface
Forms naturally 15 to 50 km above Earth’s surface
As the layer thins, holes can be created allowing larger amounts of UV radiation through which harms all life.
CHLOROFLUOROCARBONS (CFCS):
Chemicals made of chlorine and fluorine that reacts with UV radiation which ultimately destroys ozone
The chlorine atom in CFC’s will react with 100 000 ozone molecules
CFCs are found in refrigerators, aerosol cans, fire extinguishers
The hole over the South Pole was created because the ice particles in the atmosphere speeds up the reaction that destroys ozone molecules
The montreal Protocol - Healing the ozone layer
In 1987 world leaders and scientists met in Montreal QC to ban ozone depleted chemicals, 30 years later the ozone hole is slowly repairing itself.
CHANGING THE CONCENTRATION OF HARMFUL CHEMICALS IN THE ENVIRONMENT
The concentration of pollutants can be changed using different techniques:
Dispersion (spread it around)
Dilution (water it down)
Biodegradation (let nature break it down)
Phytoremediation (use plants to collect it)
Photolysis (let the sun break it down)
Dispersion:
The scattering of a substance away from its source
Dilution:
Reduces the concentration of a pollutant by mixing the polluting substance with large quantities of air or water
Dispersion and dilution may not meet government standards in leaving an area clean enough.
Biodegradation (Aka Composting):
The breakdown of materials by organisms such as earthworms, and micro-organisms like bacteria, fungi, algae and protozoa. Two types of biodegradation are aerobic and anaerobic.
Factors affecting biodegradation:
Temperature (bacteria like it warm)
Soil moisture (soil should be damp)
pH (preferably between 5 - 8)
Oxygen supply (mix the compost to introduce oxygen for aerobic bacteria)
Nutrient availability (ex. cut grass will have more nutrients than paper, however both decompose)
TIME LAPSE OF VERMICOMPOST - CHAMBER 1 PLANT MATERIAL, CHAMBER 2 CARDBOARD, CHAMBER 3 PAPER
Aerobic biodegradation:
Organisms require oxygen to degrade material
Anaerobic biodegradation:
Organisms do NOT require oxygen to degrade material
ROOTS ABSORB TOXINS IN THE SOIL
Phytoremediation:
A technique that can be used to reduce the concentration of harmful chemicals in soil and groundwater by the planting of plants that absorb or accumulate (build up) large amounts of chemicals from the soil.
The plants are allowed to grow absorbing the chemicals, then are harvested and burned or composted.
These chemicals include metals, hydrocarbons, solvents, pesticides, radioactive materials, explosives and landfill leachate.
VAMPIRES ARE PRONE TO PHOTOLYZE
Photolysis:
The breakdown of compounds by sunlight.
For example, photodegradable plastic, it is made up of chemicals that when they react with sunlight, they turn the plastic into a fine powder.
Hazardous Household Chemicals
Rather than collect and clean up harmful chemicals from the environment it is better to be proactive and not let the chemicals enter the environment in the first place.
Hazardous household chemicals includes:
household cleaners
paint and paint products
personal hygiene products
pesticides and fertilizers
pet-care products
automotive fluids
An average household has between 12L and 40L of hazardous products
These products can cause burns, heart, kidney and lung ailments, cancer, death.
Government Regulations
Designed to protect consumers and reduce risks in transporting, storing, using and disposing of hazardous materials.
Workplace Hazardous Materials Information System (WHMIS) :
Provides information on hazardous materials using symbols.
Material Safety Data Sheets (MSDs):
Detailed information sheets about hazardous products, provided by the manufacturer, the information includes:
description (describes composition, physical appearance and chemical characteristics)
precautions (how to handle said chemical)
health effects (outlines how the chemical may harm health)
first aid treatment (what to do if the chemical is some how ingested, or absorbed some how)
spill procedures (precautions that need to be taken for proper clean up)
disposal advice (how to safely dispose chemical and minimize harm to environment)
labels & New Product Regulations
For a brand new chemical product to enter the market, companies must provide information including:
intended use
physical and chemical properties
active ingredients (chemicals with biological effects)
instructions for use/safety precautions
health effects
environmental effects
toxicity to humans
first aid instructions in case of poisoning
how to safely store chemicals
To prevent accidents and unwanted injury from harmful chemicals during storage follow these rules:
Leave in original containers
Make sure they have secure lids
Place in storage areas where they cannot fall over and spill
Store in cool, dry, ventilated places away from heat
Store reactive chemicals away from each other
Transportation of Consumer Goods
Hazardous materials should be placed in the trunk
Containers should stand upright and should not be able to move
Never mix chemicals together in one container
Should be left in original container when being disposed of
Container’s WHMIS labels should be visible
DISPOSED AEROSOL PAINT SPRAY CANS
Disposal of Hazardous Chemicals
Never down the drain or into soil
Sewage treatment plants and septic tanks cannot remove these chemicals
Can contaminate drinking water, soil and air, and harm organisms if disposed of improperly
Hazardous Waste Collection Sites
These sites are used to dispose of hazardous wastes like paints, fertilizers, oils, batteries, and acids.
Materials not recyclable are labelled and sent to incineration plants