Viscosity - That's a new word
One property of fluid is how they move or flow. Why?
Viscosity :
Liquid’s internal resistance or friction that keeps it from flowing.
If a liquid has a high internal resistance (pours slowly) it's considered to have a 'thick' viscosity
If a liquid has a low internal resistance (pours quickly) it's considered to have a 'thin' viscosity
How does this happen?
The particle model of matter theory explains that particles have forces that attract one another, this force is called cohesive force. In gases this cohesive force is very weak, while in liquids this force is much stronger.
A stronger cohesive force in liquids indicates a more viscous liquid
Fluids with high viscosity do not flow as easily as fluids with low viscosity
Why care about viscosity?
Engineers must calculate how long it takes for a fluid to flow, or to fill a reservoir
Physicist must figure out how much energy is needed to pump fluids a processed fluid
Mechanics need to know how oil will flow in an engine to keep it lubricated
Understanding Viscosity and Temperature
MOTOR OIL WITH A 5W-30 RATING
As the temperature of a liquid increases, its viscosity decreases. (flows faster)
As the temperature of a liquid decreases, its viscosity increases. (flows slower)
Particles can slide more quickly when there is extra energy from heat.
Measuring Viscosity
Ramp method
Pouring a fluid down a ramp and timing how long it takes to get to the bottom
Able to test different fluids, or same fluids at different temperature, or both!
Flow Rate:
The amount of a substance moving in a given amount of time
Flow Rate (FR) = Volume (ml)/time (s)
Slower flow rate indicates the fluid has a higher viscosity
Faster flow rates indicate the fluid has a lower viscosity
Couple questions for you!
What is the flow rate of a substance that takes 14 seconds for 500 ml to flow?
What is the flow rate of a substance that takes half a minute for 0.5 L to flow?
Density of Fluids
Density :
The amount of mass occupying a certain amount of volume determines an object’s density.
Not all substances have the same density, because different substances are made of different particles (particle theory).
This results in different densities due to the different particles!
If an object has a larger density than a solution, it will sink.
If an object has a smaller density than a solution, it will float.
Calculating Density
Density (D)= Mass (m)/ Volume (v) or D= m/V
For liquids the units for density are grams per millilitre (g/mL) or kilograms per litre (kg/L).
For gases it is grams per centimeter cubed (g/cm³).
You may notice that the units for concentration and density are very similar, and that’s because they are. The basic difference is concentration pertains to solute amount in a solvent, where density refers to amount of mass inside a volume.
Some questions for you!
Substance A - Has a mass of 40 g and a volume of 100 ml, what is it’s density?
Substance B - Has a mass of 250 g and a volume of 130 ml, what is it’s density?
Substance C - Has a mass of 0.2 kg and a volume of 0.2 L, what is it’s density?
Hydrometer:
An instrument that measures the density of a liquid
Galileo’s Thermometer
A hydrometer is a device for measuring the density of liquids
Each temperature bulb acts like a hydrometer and floats to the top when the water’s density is greater than that of the bulb
A Recap on Density & Temperature
Viscosity changes with temperature
Temperature increases, viscosity decreases = fluid flows easier
Temperature decreases, viscosity increases = fluid flows slower
Think - what flows more melted butter, or room temperature butter?
How to change density
Two ways to change density:
Change the size of a substance’s volume (Temperature does this - think hot air balloon)
Change the mass of particles in that substance (Adding salt to water increases the solution’s mass)
Changing the Density by adding energy
Adding energy to particles makes particles move faster, which makes them move further apart
If particles move farther apart it will increase the volume, but since the number of particles stays the same, the mass stays the same!
This results in the density decreasing.
What would happen if you were to take away energy from particles?
Changing the Density by changing Concentration
Adding more solute to a solution would increase the number of particles which would then increase the mass.
Increasing the mass would increase the density
What would happen if you were to reduce the amount of solute in a solution?
The Particle Model of Matter as an Explanation for Density Changes
Buoyancy
Buoyancy :
Tendency of an object to float when placed in a fluid
When an object is in a liquid, the force of gravity pulls it down.
The liquid however exerts an opposite force that pushes the object upward
This is called the buoyant force!
Buoyant Force :
Upward force that a fluid exerts on an object; opposite to the pull of gravity on an object in a fluid.
If the buoyant force of an object is greater than the force of gravity, the object will float.
In other words, if the density of the liquid is greater than the density of the object, the object floats.
Neutral buoyancy :
Is when the force of gravity is equal to the buoyant force
Heavier object will sink…Duh
Gravity puLLS an object down. The more mass the object has, the more it will be pulled down.
• When you dive in water, you’re able to go downward because the force of gravity and force of leg kicking is greater than the buoyant force of the water
To move upward, the buoyant force of water and leg movement is greater than the force of gravity!
Buoyancy in the Real World
A fully loaded boat that crosses the Atlantic ocean and enters the fresh water of the St. Lawrence river will sink lower because the density of the salt water is greater than the density of the fresh water.
Same thing occurs from cold water in the North to warm water in the south…the ship sinks. Why? Temperature has an effect on density!
Because of density variations among different oceans, cargo ships have a Plimsoll line. It helps show water density and how heavy a ship can be loaded in different water conditions (much like a hydrometer)
Hot air balloons are buoyant as well, they float on the layer of cold air below it.
Compression
In the particle model,the amount of space between particles depends on what state (S,L,G)
A ball can compress…Why?
Air is inside the ball. It has lots of space between the particles, therefore can be compressed the most! As a result, when the ball hits the ground, the ball’s shape deforms.
Liquids and solids have very little space between particles and are considered incompressible.
Compressibility :
The extent to which a substance (gases) can be compressed; objects under compression tend to deform in shape (ex. Soccer ball)
More compression takes place in gas than liquid because there is more space between the gas molecules than the liquid molecules.
Incompressible :
Not capable of being compressed (ex. Liquids).
Pressure in Fluids- Pascal’s Law
Blaise Pascal, a mathematician was curious how force is applied to a fluid
Discovered a relationship between water pressure and depth
The greater the depth, the greater the pressure because the weight at the top puts more pressure on the bottom.
Pressure :
Measure of the amount of force applied to a given area; measured in Pascals (Pa).
1 Pa is very small. It’s the weight of your loose leaf paper on your desk!
Pascal’s Law :
When pressure is applied to a liquid in a container, the pressure and force is transmitted equally and undiminished throughout the liquid; enclosed liquid transmits pressure equally in all directions.
Pascal’s Equation for Pressure
Blaise Pascal also found there was a mathematical relation between force and area:
Pressure = Force/ Area or P = F/A
Units: 1N/m² = 1 Pa
1000 Pa= 1 kPa
Example - You have a force of 10N on an area of 2m²:
P = F/A
= 10 N/ 2m²
= 5 N/ m²
= 5 Pa
Think!
Why does a sharp knife take less effort to cut through material?
Why does a garden hose shoot water further when you put your finger over the spout?
Barometer :
An instrument which measures the pressure of air in the atmosphere
Pressure, depth and scuba!
SCUBA – Self Contained Underwater Breathing Apparatus
Scuba gear allows divers to breathe underwater using pressurized air tanks
At greater pressure, nitrogen gas dissolves in our blood stream, this can have damaging affects.
If a diver ascends too quickly from the depths and the pressure decreases rapidly, the dissolved nitrogen bubbles out quickly and collects in other body parts, this causes great amount of pain. This is called “the bends.”
This is why a diver needs to come up to the surface slowly; for the extra gas leaving the body slowly as water pressure decreases.
To Summarize Pascal’s work:
Pressure is a force pushing on a surface (can be solid, liquid or gas)
The greater the depth of a fluid, the greater the pressure
Force is spread out equally in all directions in a closed system and contained system
Systems that use Pressure
Hydraulic System :
A system that uses a liquid (usually water or oil) under pressure to transfer forces and move loads. Works according to Pascal’s Law !
Piston:
A disk or short cylinder fitting closely within a tube in which it moves up and down against a liquid or gas
How it works
The small piston is the input disk where force is applied
The big piston is the output disk where force lifts or pushes an object
The amount of pressure in the small piston is the same as the large piston
Pneumatic System :
A system that uses gas under pressure to move loads; device that uses gases in a confined space to transfer forces; works according to Pascal’s Law.
Technologies Based on Flow Rates and Moving Fluids
Pump :
Device that moves a fluid through or into something
3 common kinds of pumps:
piston pumps (bicycle pump)
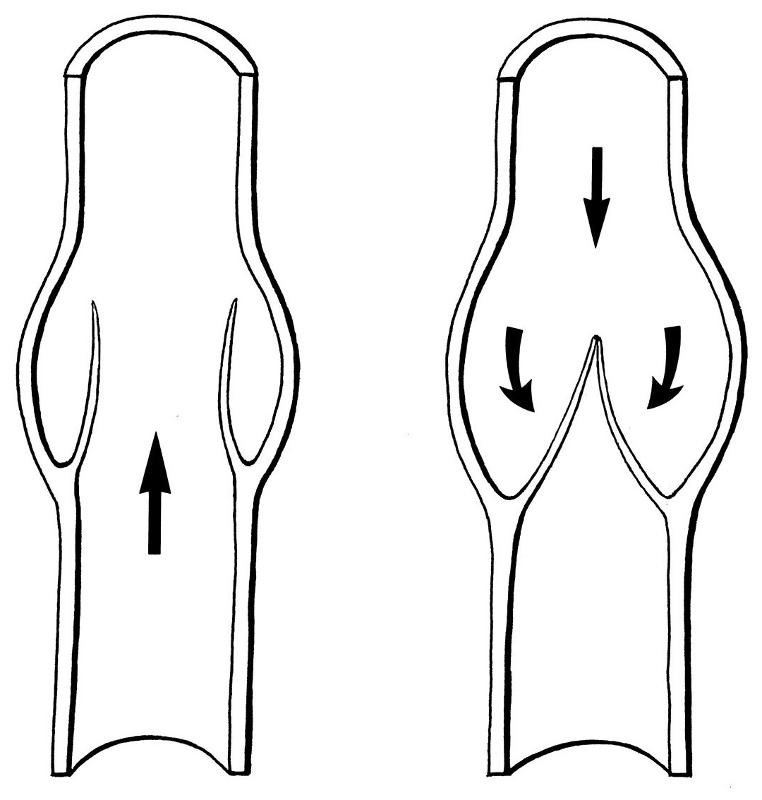
diaphragm pumps (aquarium air pump, the heart)
Archimedes screws (irrigation, mining operations)
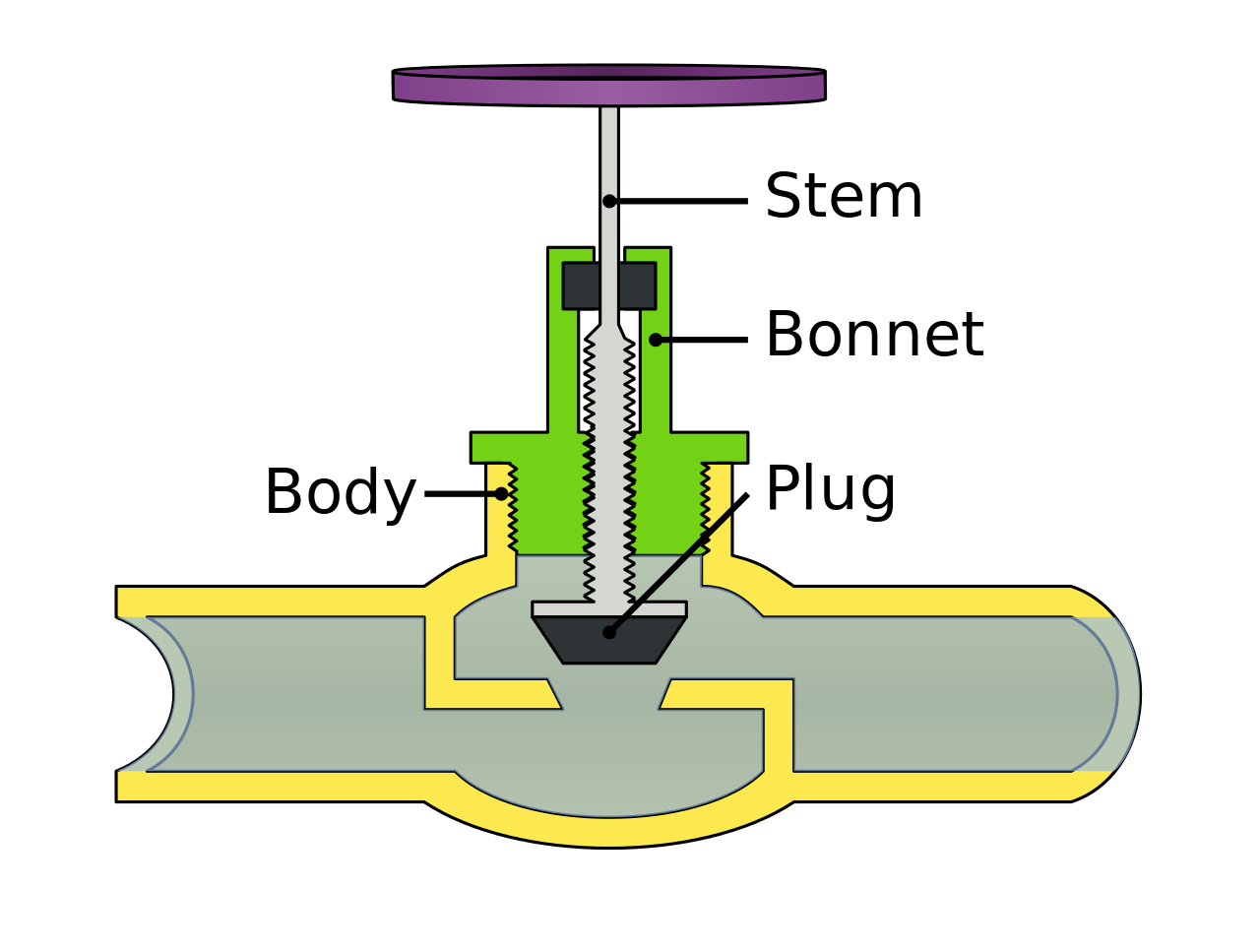
Valves :
A device that controls the flow of fluids. Specifically start/stop flow, change speed of flow, control direction of flow.
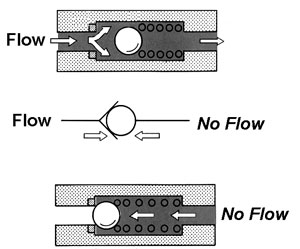
Check valves
Stop valves
Plug Valves
Types of valves:
Check valves (control direction)
heart
veins
one way valves
Stop valves (stop the flow, on or off)
butterfly valve
ball valve
Plug valves (regulates pressure/flow)
globe valve
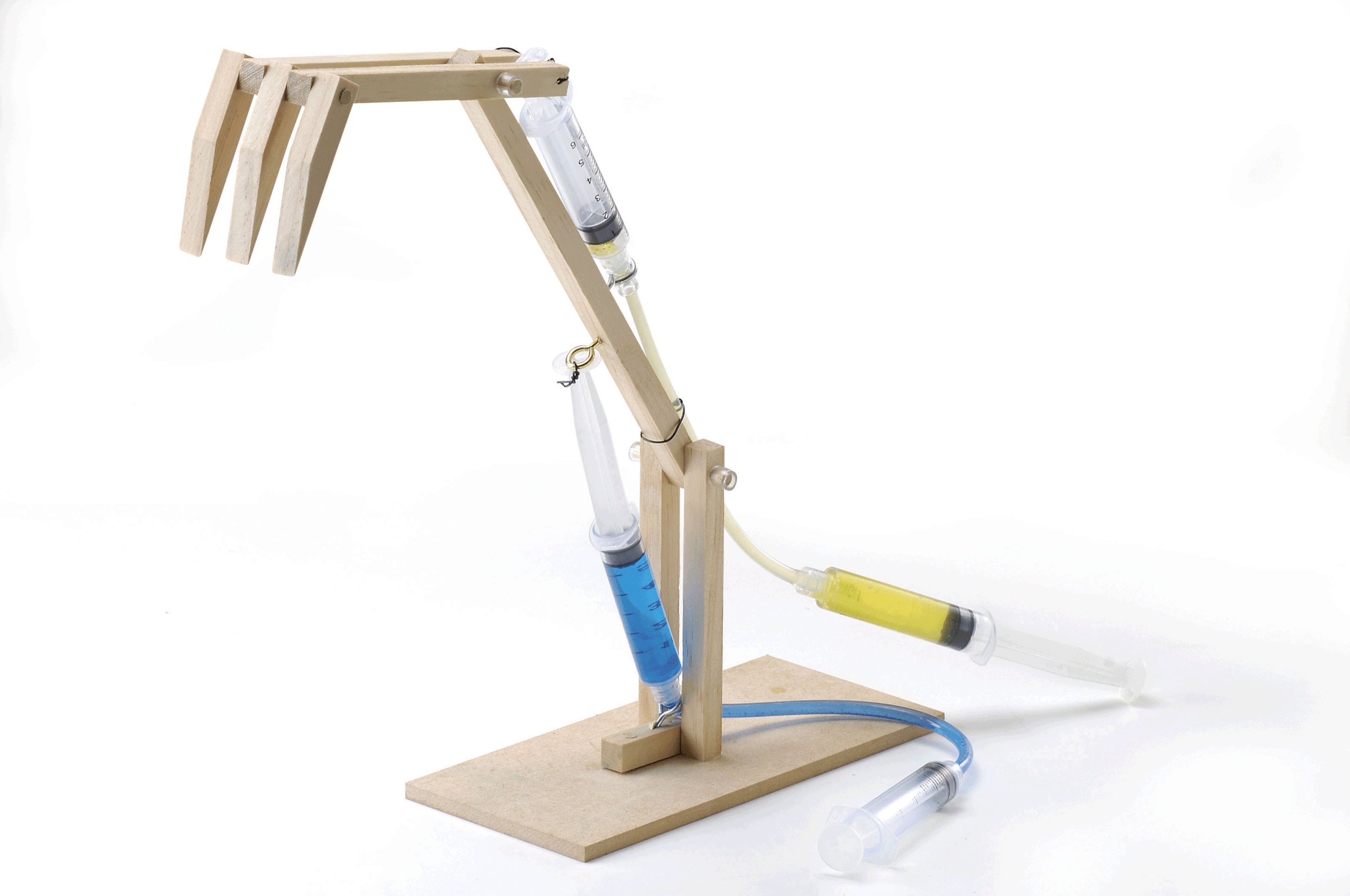
Designing a Working Model of a Fluid-Using Device
How a Submarine Works
To float, the submarine fills the ballast tanks with air which decreases the overall density on the submarine to less than that of water.
To dive, air is released from the ballast and they are then filled with water; the density of the submarine is now greater than that of the water.
To resurface, compressed air is forced into the ballast tanks and that pushes the water out, and again decreases the density so the submarine can surface.