Mix and flow of matter
The Many Uses of Fluids
Printable Notes
Fluid :
Anything that has no fixed shape and can flow (usually a gas or liquid); takes the shape of its container.
Slurry:
A mixture of water and solids. It is a form of a fluid.
Dirt and water
Cement
Hair gel
Oil sand and water
Fluids Make it Easier to Use Materials
Fluids are used in industrial processes such as mining oil sands
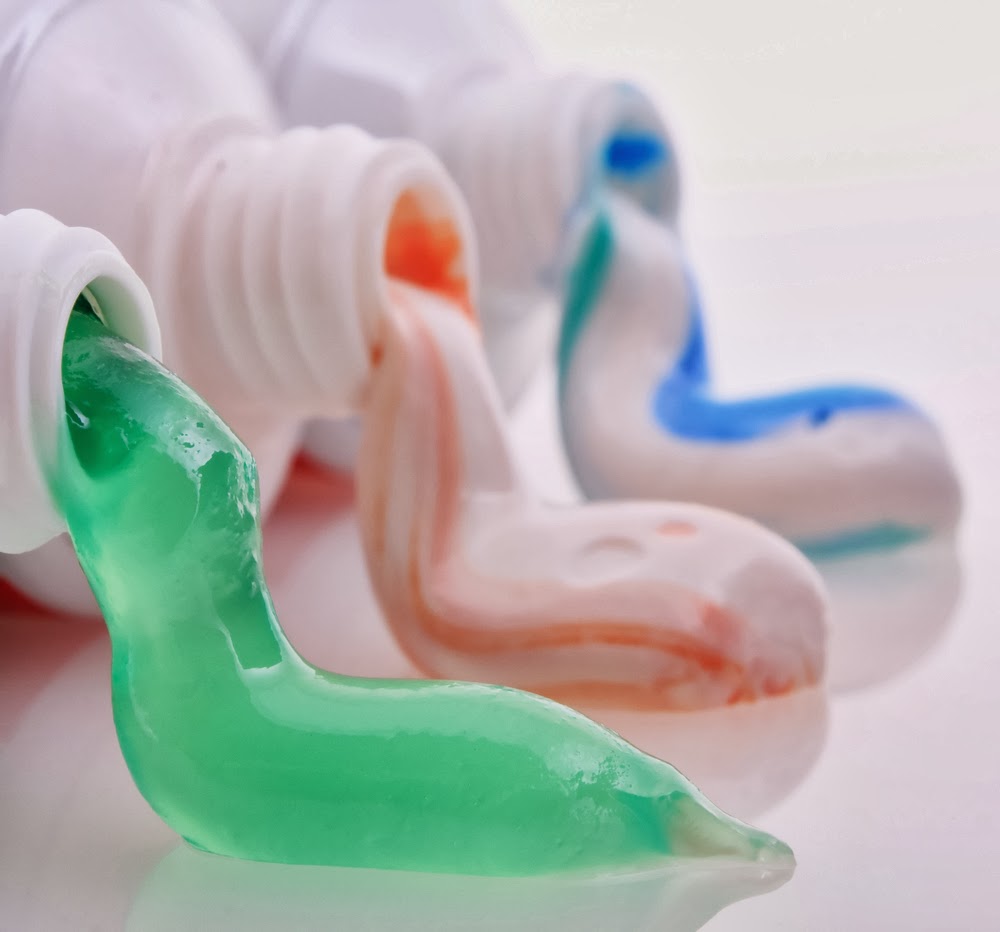
Fluids have the ability to spread or flow and carry other materials.
Fluids can be a combination of different materials. For instance toothpaste contains powdered material, detergent, fluoride, binders, coloring, and flavoring
MOLTEN METAL
Useful Properties of Fluids
Fluids are involved in transporting, processing, and using materials.
Some solids are originally prepared as fluids (ex. Glass or steel).
By understanding the properties of fluids, people can design technological devices that use these properties
An Example of How Fluids are used in everyday life
States of matter
Recall from grade 7 the states of matter:
Solid :
Keeps its shape and keeps its total volume
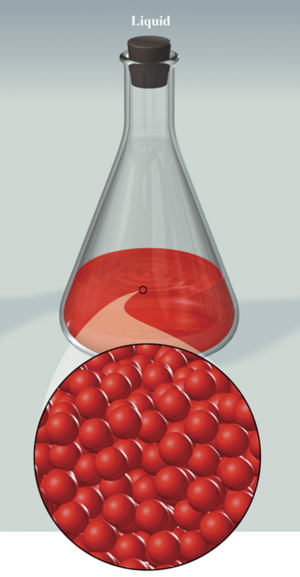
Liquid :
Takes the shape of the container and keeps its total volume
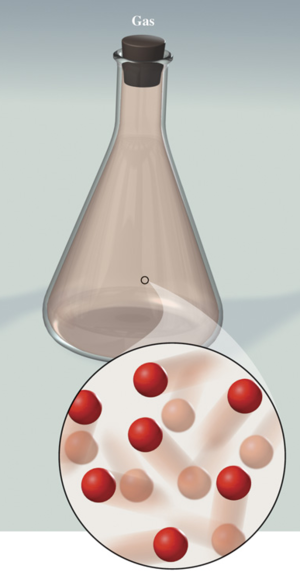
Gas :
Takes the shape of its container and changes volume to fill its container
What about transitions from state to state?
Gas to liquid - condensation
Gas to solid - deposition
Liquid to gas - evaporation
Liquid to solid - freezing
Solid to liquid - melting
Solid to gas - Sublimation
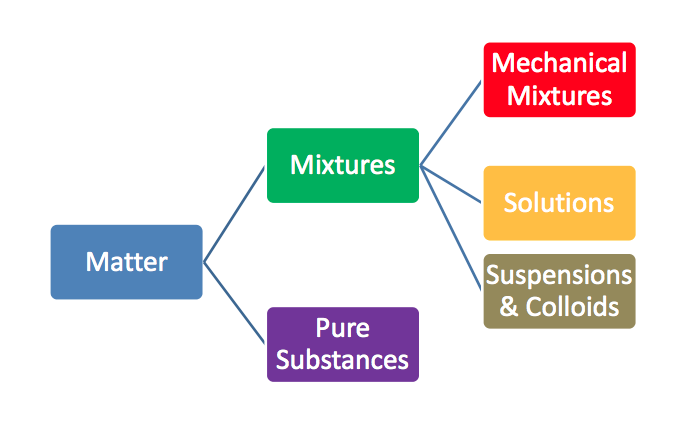
Pure Substances & Mixtures
Matter is divided up into two classifications
Pure Substances :
Made of only one kind of matter; cannot be separated into different substances. Ex. Gold, aluminum foil
Mixture :
Made up of combination of different substances Ex. Saltwater, oreos, air, pancake mix.
Mixtures can be broken up even further!
Mechanical mixture :
A mixture that you can see different substances that make up the mixture. Also called a heterogeneous mixture.
wet cement
salsa
porridge
Solution :
A mixture of 2 or more pure substances that looks like one substance. Also called a homogeneous mixture.
syrup
soda pop (before opening can)
salt water
Suspension :
A cloudy mixture in which droplets of one substance are held floating within another substance. If left undisturbed the mixture will separate into its individual parts.
muddy water
orange juice with pulp
salad dressing
ketchup or mustard
Colloid :
A cloudy mixture in which tiny particles of one substance are held floating within another substance. If left undisturbed the particle stay mixed and will NOT separate out into individual parts.
homogenized milk is a colloid of tiny cream droplets in whey
hand cream
cytoplasm inside animal cells
How can we figure out if something is a pure substance or solutions?
Paper chromatography can be used to determine if fluids are pure substances or solutions.
Filter paper is put in solution.
Pure substance- fluid travels up one level
Solution - fluid travels up many level
Concentration & Solubility
Solvent :
A substance that dissolves a solute to form a solution(ex. Water, known as the 'universal solvent').
Solute :
A substance that dissolves in a solvent to form a solution.
Solution :
Dissolving one substance into another creates a solution, the solute particles are attracted to the atoms of the solvent. A solution should look like one fluid, no floaties!
Measuring Concentration
Concentration :
The amount of solute (g or kg) dissolved in a specific amount of solvent (mL or L) in a solution; written as g/L or kg/L. Means amount of solute/amount of solvent.
We use terms like 'concentrated' and 'diluted' to describe the solutions:
In a concentrated solution there are large amounts of solute in a solution
In a diluted solution there are small amounts of solute in a solution
Different types of concentrations
g/L
Parts per million (PPM) - Very small amount
Parts per billion (PPB) - Very very small amount
Comparing Concentrations (yay Fractions)
Is the numerator in (g) units - If not, convert it to (g)
Is the denominator in (L) units - If not, convert it to (L)
If the numerator & denominator have the proper units then divide
Compare different concentrations
Try the following:
Compare a solution where 25g of salt is added to 100ml of water, to a solution of 10g salt added to 50ml
Compare a solution where 0.5 Kg of salt is added to 1L of water, to a solution of 1kg salt added to 1.8L
Compare a solution where 0.25 Kg of detergent is added to 600 ml of water, to a solution where 6.5 Kg of detergent is added to 60 L of water
Saturated and Unsaturated Solutions
Unsaturated Solution :
A solution in which more solute can be dissolved at a given temperature
Saturated Solution :
A solution in which NO more solute can be dissolved at a given temperature
Solutions do not have to be made up of only liquids
Ex. carbonated pop
What happens if you add more solute to an unsaturated Solution?
Comes to a point where it becomes saturated
If you change the pressure, volume or temperature, it can become ‘super saturated’
ADDED PRESSURE INCREASES THE AMOUNT OF SOLUTE IN A SOLUTION
Solubility :
The ability to dissolve; the mass of solute that can dissolve in a given amount at a given temperature.
Saturation Point :
The point at which NO more solute can be dissolved at a certain temperature
Factors Affecting Solubility
Solubility depends on 3 factors.
Type of solvent
Type of solute
Temperature
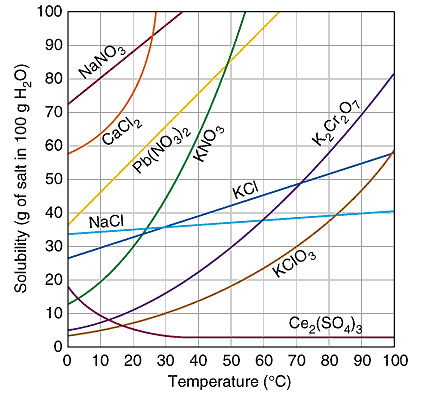
SOLUBILITY OF DIFFERENT CHEMICALS, NaCl IS SALT, NOTICE HOW IT BECOMES MORE SOLUBLE AS YOU INCREASE TEMPERATURE
Aqueous Solution :
When water is the solvent in the solution. Water is sometimes referred to as the “universal solvent” because it can dissolve so many things.
Solubility Changes with Temperature
For most substances, solubility increases as the temperature of the solvent increases
Ex. at 25 degrees Salt- 36.2g/100ml water is saturated
Ex. at 100 degrees Salt- 39.2g/100ml water is saturated
BLUE GREEN ALGAE FLOURISHES IN WARMER WATER, IT CAN BE TOXIC IN LARGE AMOUNTS
Thermal Pollution
Decrease in solubility of gases have a serious effect on the environment
Water drawn from a lake as a coolant. When it goes back to the lake and it is not treated, it is warmer than it left
The Particle Model of Matter and the Behaviour of Mixtures
Some history...
Ancient Greek Democritus said all matter was made of small, invisible, indivisible particles
Nobody believed him for 2000 years!
1808- John Dalton believes him and makes his atomic theory
Particle Model of Matter :
A model that explains the behaviour of solids, liquids, and gases; it states that all matter is made up of tiny moving particles that attract each other and have spaces between them.
Four Main Points of the Particle Model of Matter:
1. All matter is made up of tiny particles.
Different substances are made up of different particles.
There are more particles in a given volume of solid than there are in the same volume of liquid or gas.
How big is a particle? In one snowflake there are 1018 particles.
2. The tiny particles are always moving and vibrating.
For solids, the particles vibrate in one place.
For liquids, the particles slide around and over each other.
For gases – the particles move as far as the space they are in allows.
3. The particles in matter maybe attracted to each other or bonded together.
Some particle (like water) have more attraction to particles than others (like salt).
4. The particles have space between them.
Think like marbles & sand.
How the Particle Model Explains Mixing Substances.
If the 2 substances with different size particles are mixed together, the smaller particles will fill spaces between the larger particles.
The attraction of particles helps in dissolving.
Dissolving
3 factors affect how quickly a solute will dissolve in a solvent:
Temperature – increasing the temperature causes the particles to move faster and bump into the solute particles faster.
Surface Area – small pieces of solute dissolve more quickly than large pieces because there is a greater surface area where the solvent can make contact.
Stirring – moves all the particles around, so the solvent particles bump into the solute particles.
What if substances do not mix well?
SURFACTANT MOLECULE
How do we clean oil stains on clothes when water does not dissolve oil? We use soap!
Soap (also known as detergent) is a substance that can remove dirt from a fabric with the help of surfactants which are particles that attach themselves to the dirt and water.
Surfactants have two ends, a hydrophobic and hydrophilic end.
The hydrophobic end attaches to oils and dirt
The hydrophilic end attaches to water.
Detergents also carry phosphates to help break up dirt and maintain the pH of the solution. But when detergent is washed down the drain and enters nearby lakes and rivers it can have a polluting effect by causing excessive growth of algae which deprives fish of oxygen, effectively killing fish populations.